
Bioanalysis of albumin drugs
There are bound and free forms of albumin drugs in the blood, which need to be separated by equilibrium dialysis or ultrafiltration and measured separately. Chengyao team has experience in bioanalysis of many albumin drugs such as paclitaxel and docetaxel.
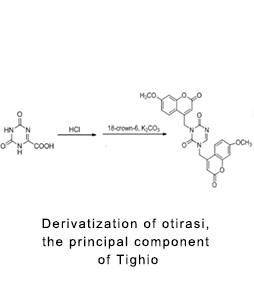
Derivative bioanalysis
The sensitivity, chromatographic retention, mass spectrometry response and stability of the drug to be tested were improved by chemical modification. The Chengyao team has completed the derivative biological analysis of more than 10 clinical studies on Teggio capsule, captopril, estradiol, calcitriol, bisphosphonic acid drugs.
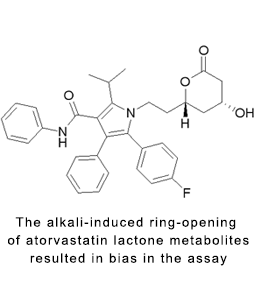
Bioanalysis of unstable drugs
The structure of the compound has lactone, ester bond, double bond isomerism and other structures, and needs to control temperature, pH, light and other conditions. Experience: There are more than 30 clinical studies on lactones (statins, camptothecin) and ester bonds (foxapyrtam, aspirin).
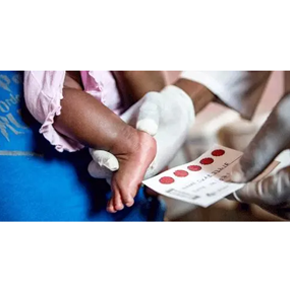
Biological analysis of dry blood spot samples
It can be used in infant clinical PK research to avoid venous blood collection. Dry blood spot tablet (DBS) was used to detect drug concentration in filter paper by mass spectrometry after fingertip or heel blood of infants. Chengyao team completed the biological analysis of a new drug infant clinical study in China.
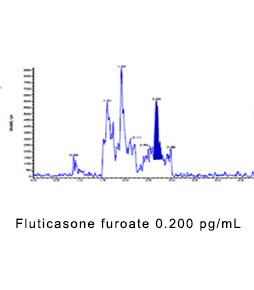
Ultra-sensitive biological analysis
Our team has developed dozens of ultra-sensitive (pg/mL) bioassays and applied them to clinical and non-clinical studies of very low doses of drugs, inhaled formulations, and local administration in vitro.
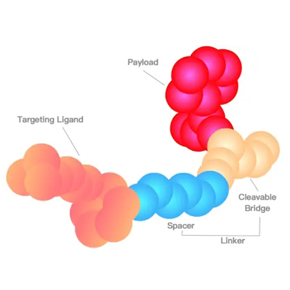
Bioanalysis of polypeptide and PDC drugs
Polypeptide drugs have some problems such as non-specific adsorption, poor chromatographic retention and difficult extraction. PDC bioanalysis is affected by stability problems. Chengyao team has developed dozens of biological analysis methods for polypeptides and PDC drugs.
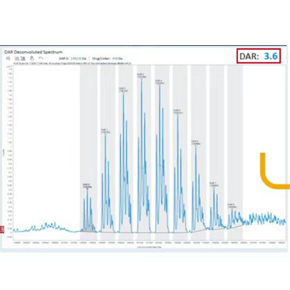
ADC drug biological analysis, DAR value analysis
Chengyao also has a large and small molecular analysis platform, which can meet the ADC drug and payload detection. The high resolution mass spectrometry platform can detect DAR values.
Mass spectrometry analysis of nucleic acid drugs
Nucleic acid drugs are difficult to extract, non-specific adsorption is serious, chromatographic peak pattern is poor, and biological analysis is difficult. Chengyao has a complete solution for nucleic acid drug quantitative bioanalysis. And experience in liposome analysis. It can meet the requirements of nucleic acid drug registration research.

Protease hydrolysis mass spectrometry
After proteolytic hydrolysis, the characteristic peptides were quantitatively analyzed to solve the problems of poor selectivity, difficult preparation, high cost and long delivery time of enzyme-linked immunoreagents. It passed the verification of the ability of mass spectrometry to detect bevacizumab.
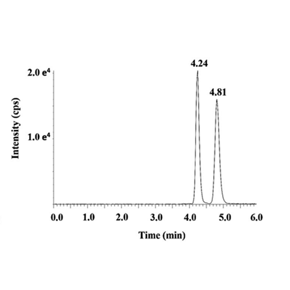
Ultrafine chromatographic separation of positive phase chiral drugs
The determination of L-scopolamine in plasma by mass spectrometry after chiral resolution of normal phase chromatography was developed. The separation efficiency was much better than that of conventional reverse chromatography, and the analysis time was 5 minutes.